BRCA1 and BRCA2 mutation detection
BRCA1 and BRCA2 mutation detection
Test Overview BRCA1 and BRCA2 (Breast Cancer genes 1 and 2) are human genes that produce tumor suppressor proteins. These proteins play important role in the repair of damaged DNA before a cell divides during natural process of cell division.
Test Overview
BRCA1 and BRCA2 (Breast Cancer genes 1 and 2) are human genes that produce tumor suppressor proteins. These proteins play important role in the repair of damaged DNA before a cell divides during natural process of cell division.
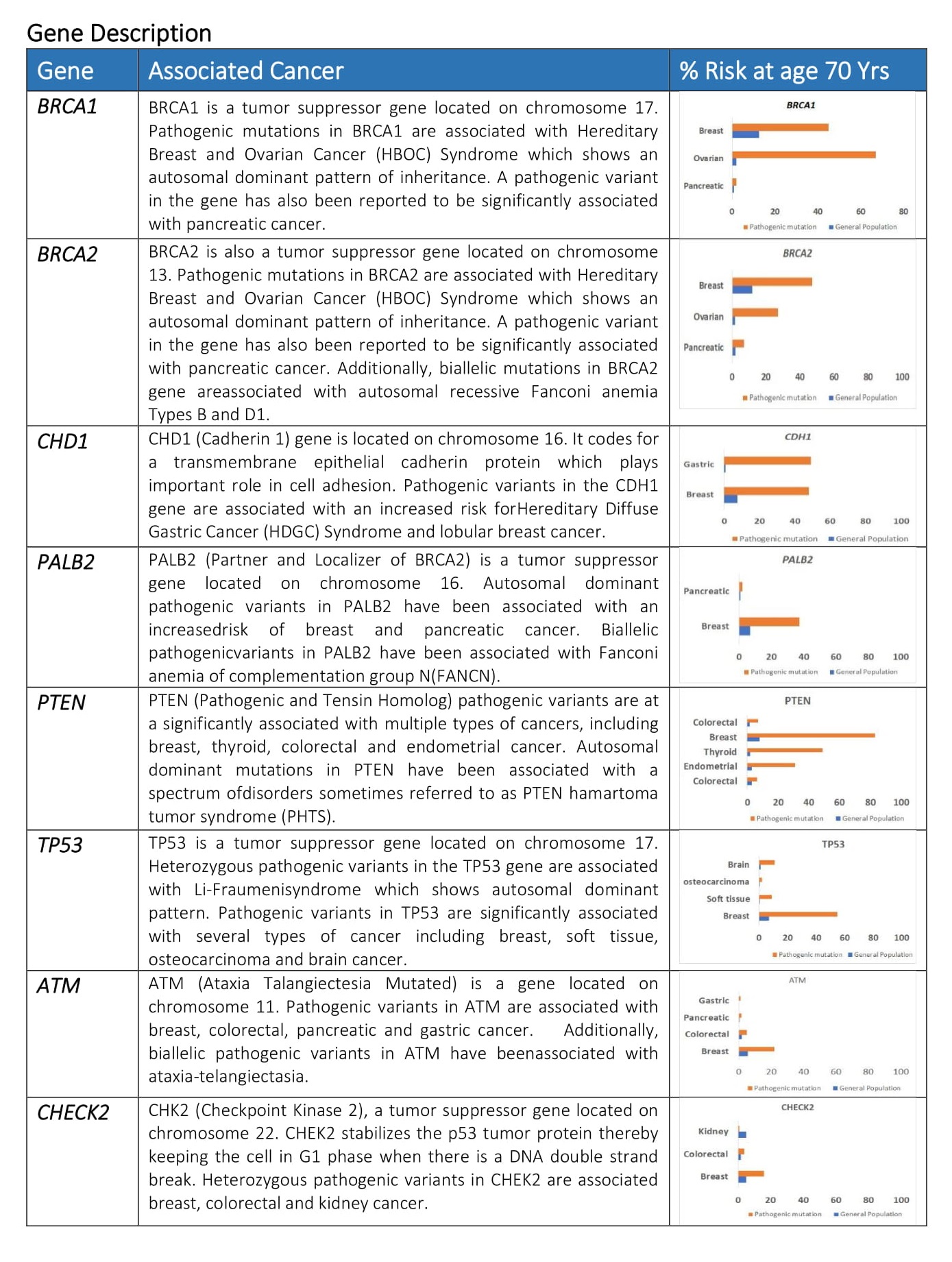
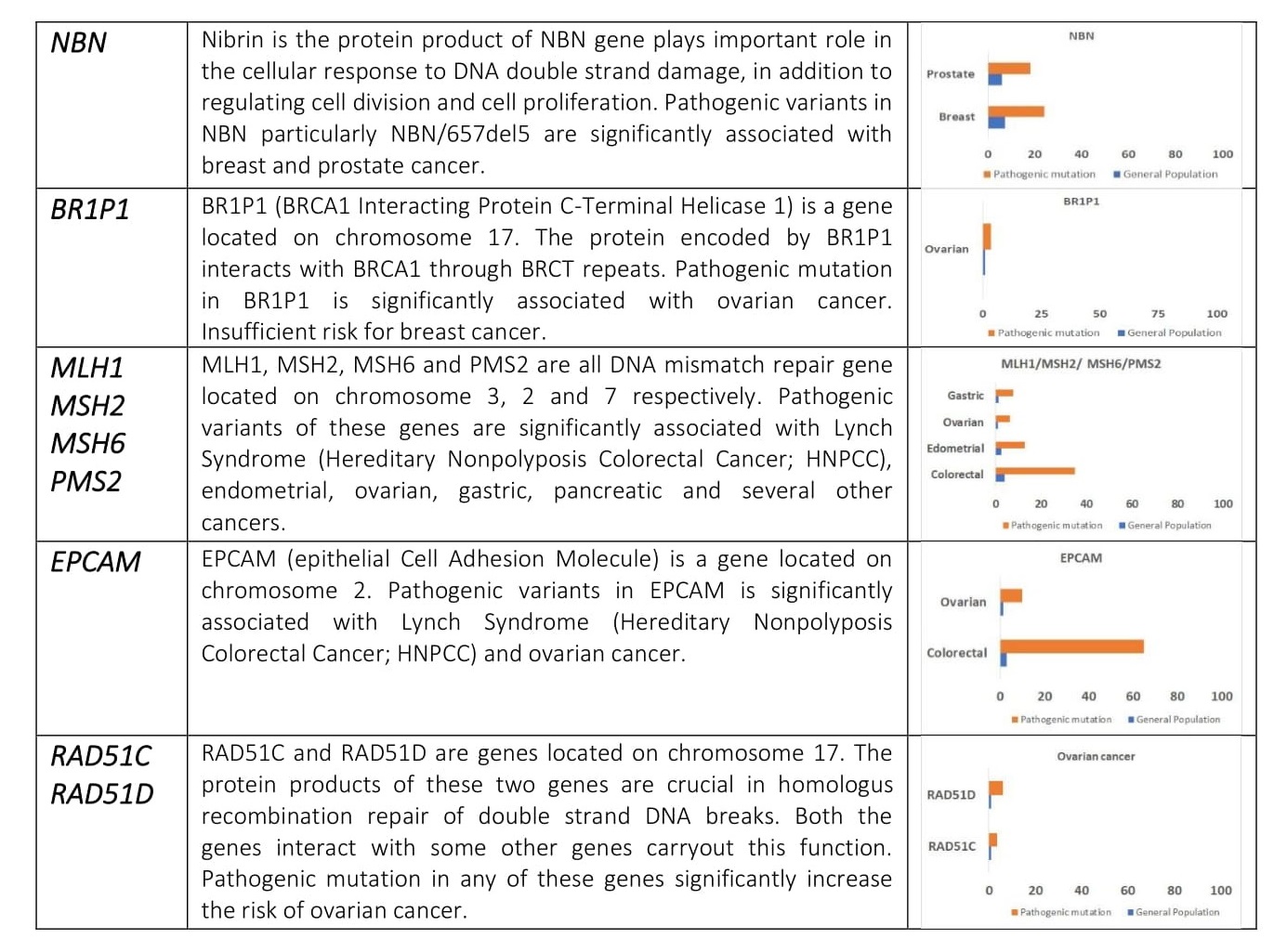
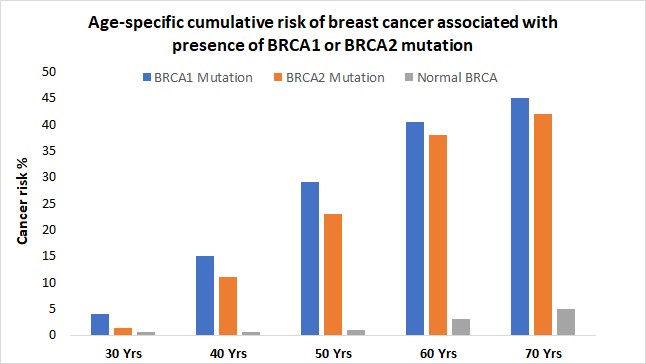
When either of the genes is mutated its protein product no longer functions properly and DNA damage may not be corrected properly. As a result, cells are more likely to develop additional genetic alterations that can ultimately lead to cancer. Inherited mutations in BRCA1/2 are associates with an increased risk of breast and ovarian cancer in women. Somatic mutations in BRCA1/2 are also common in several other cancer types. Individuals with inherited mutation in BRCA1/2have a condition called Hereditary Breast and Ovarian Cancer Syndrome (HBOC). They tend to develop breast and ovarian cancer at younger ages than people who do not have these mutations. Women with HBOC also have a higher risk of developing fallopian tube and primary peritoneal cancer. About 12% of women in the general population will develop breast cancer sometimes during their lifetime. By contrast, about 72% of women who inherit a harmful BRCA1 mutation and about 69% women who inherit a harmful BRCA2 mutation will develop breast cancer by the age of 80. Breast cancer is the most common type of cancer among women worldwide and is second leading cause of death in women.
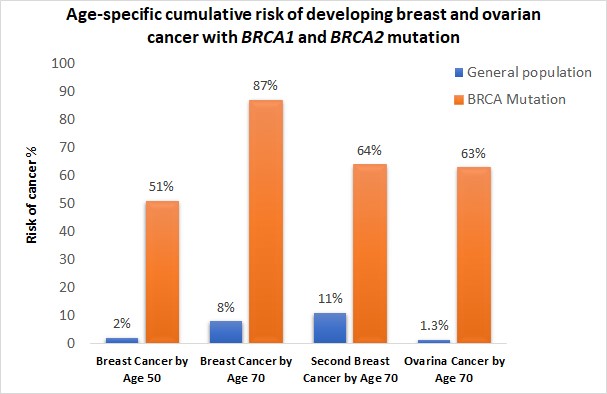
- Breast cancer diagnosed before the age of 50 years
- Cases of ovarian cancer
- Cancer in both breasts of the same women
- Multiple breast cancer in the family
- Both breast and ovarian cancers either in the same women or the same family
- Triple negative breast cancer, particularly when diagnosed before age 60 years
- Cases of male breast cancer
- Ashkenazi Jewish ancestry
- A previously identified BRCA1 or BRCA2 pathogenic variant in the family
- A personal or family history of pancreatic cancer, aggressive prostate cancer or metastatic prostate cancer at any age
Most experts in the field of oncology agree that mutation testing of individuals who do not have cancer should be performed only when the person’s individual or family history is suggestive of the presence of a harmful mutation in BRCA1 or BRCA2.
The United States Preventive Services Task Force (USPSTF) recommends that women with personal or family history of breast, ovarian fallopian tube or peritoneal cancer be evaluated to see if their family history is associated with increased risk of a harmful mutation in any of these genes.
Several screening tools are available to help primary health care physicians to make decisions more easily and reliably regarding when to refer patient for genetic testing of BRCA1 and BRCA2. The Six-Point Screening Tool is useful as a simple tool. It can be administered with paper and pencil at primary health care clinics in a low resource setting. Several other online screening tools such as, Referral Screening Tool (https://www.breastcancergenescreen.org/) or Tyrer-Cuzick (http://www.ems-trials.org/riskevaluator/)can also be used. However, these tools will require some level of IT facility and skills. Routine screening and genetic testing are NOT RECOMMENDED among the general population without a family history suggestive of BRCA1 or BRCA2 mutation.
Analysis methodGenomic DNA is extracted from blood, cell lines or formalin fixed, paraffin-embedded (FFPE) samples using standard methods. The entire coding region and intronic flanking regions of the BRCA1 and BRCA2 genes are then amplified using Ion AmpliSeq Panel (Thermo Fisher Scientific, USA) consisting three primer pools. The resulting amplicons are pooled and ligated to adapters and barcodes. Multiplexed barcoded libraries are then amplified by emulsion PCR on Ion Sphere Particles (ISPs) using OT2 kit according to the manufacturer’s protocol. Finally, the sequencing is performed on Ion GeneStudio S5 Next Generation Sequencing platform using Ion 520 chip.
Sequencing data are analyzed by Ion Reporterä Software. The automated bioinformatics analysis within the software provides a full set of basic variant annotations such as, SNVs (Single Nucleotide Variations), MNVs (Multiple Nucleotide Variations), InDels (insertions or deletions) and whole-exon, multiple-exon or entire gene aberrations with high sensitivity and specificity. The Ion Reporterä is powered with easy cross-reference capability to ClinVar, a leading archive of human genetic variation interpretation.
Sample requirementFor germline mutation: Blood (collected in EDTA tube) or buccal cells
For somatic mutation: Formalin Fixed Paraffin-Embedded (FFPE) tissues from tumor samples
PreparationNo preparation needed
Instrument platformIon GeneStudio S5, Next Generation Sequencing Platform (Thermo Fisher Scientific, USA).
Interpretation of BRCA1 and BRCA2 genetic testingThe result of BRCA1 and BRCA2 genetic testing has several possible outcomes:
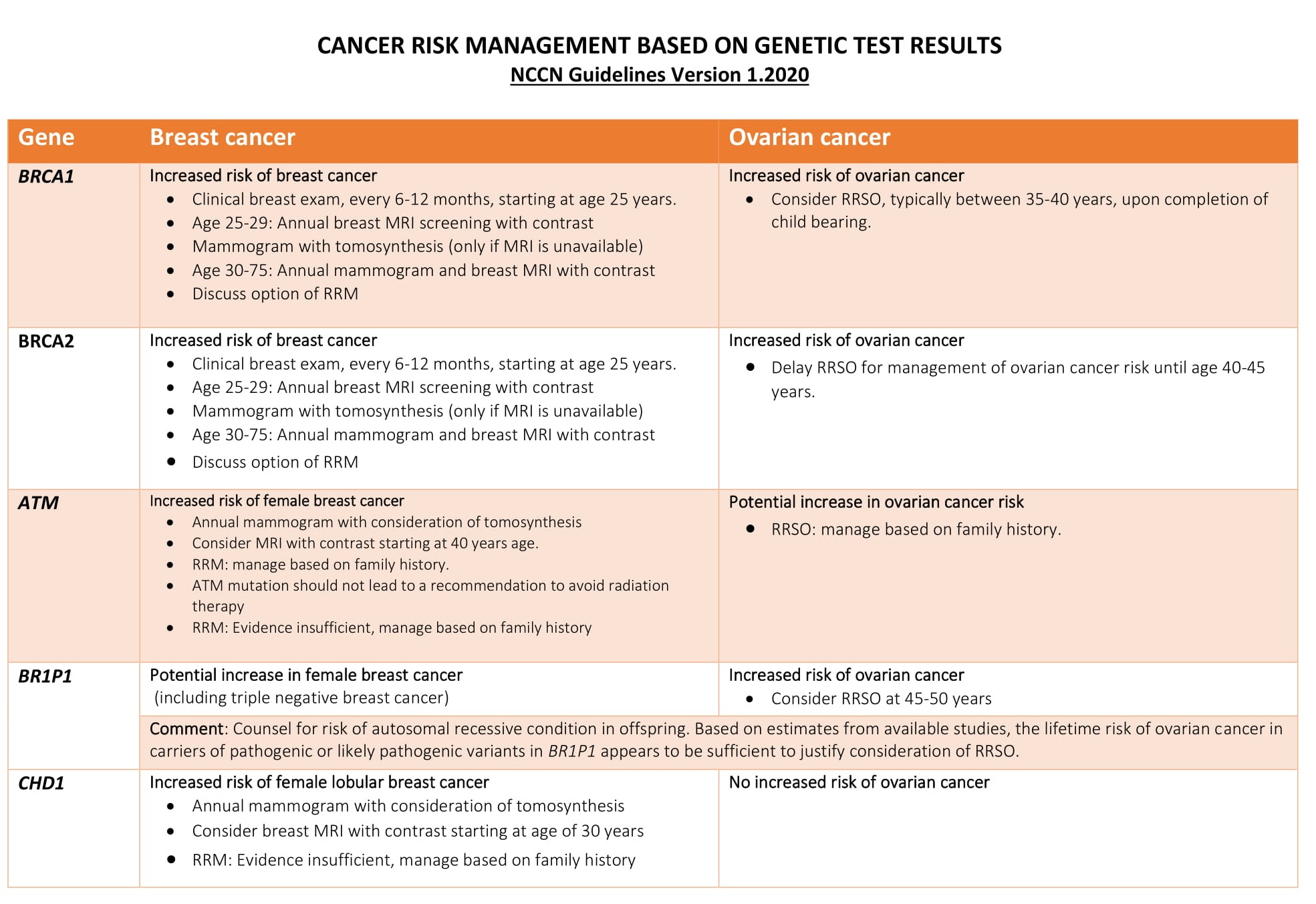
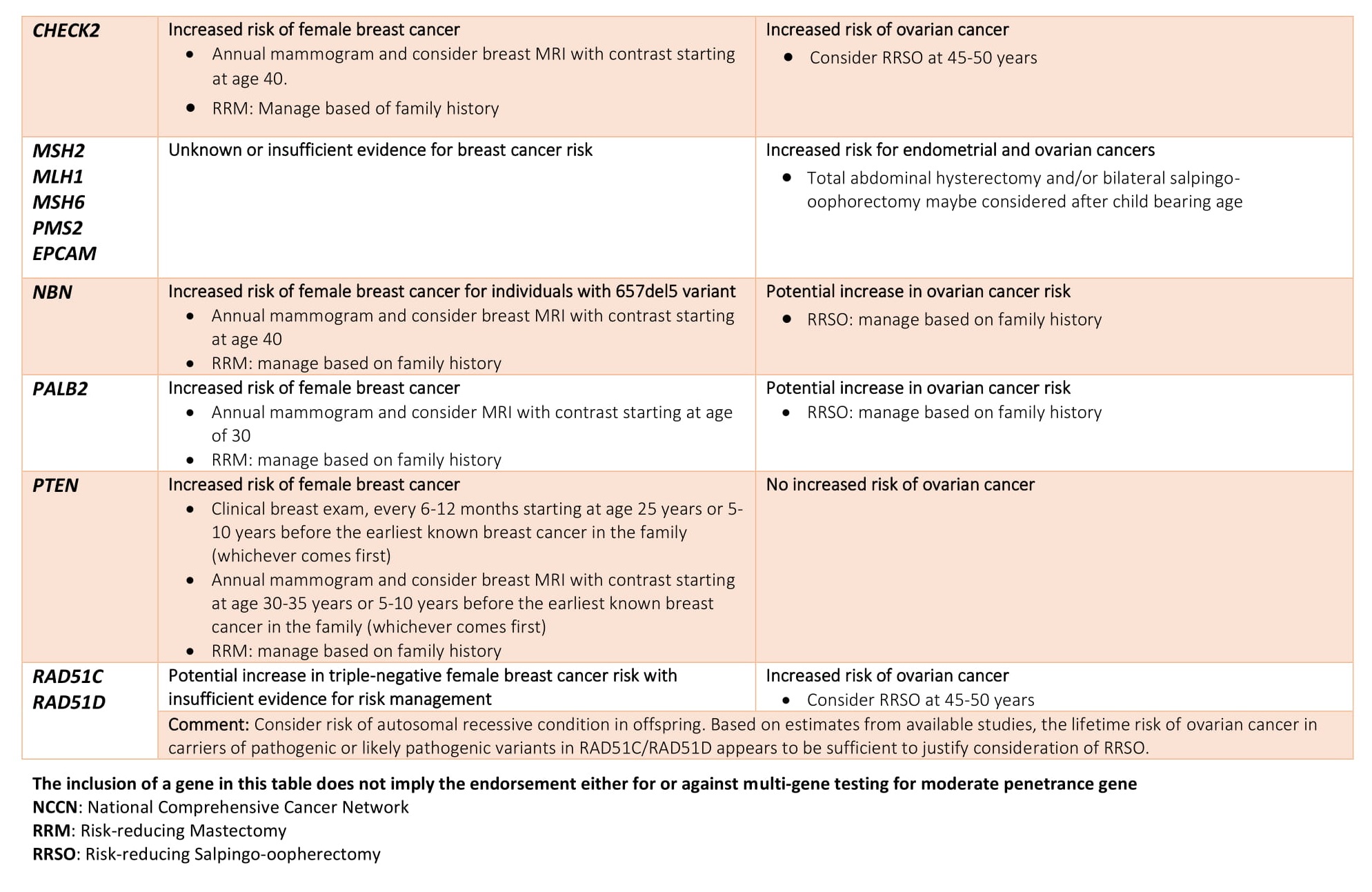
- Positive result: A positive test result indicates that the tested individual has inherited a harmful pathogenic mutation in either BRCA1 and BRCA2 This means the person has an increased risk of developing breast, ovarian and certain other cancers. Genetic counseling and consultation with the physician are recommended to these group for risk reducing interventions. However, a positive test result does not always mean that you will have cancer. It only means that you have a harmful BRCA1 or BRCA2 mutation and at an increased risk of developing early onset breast or ovarian cancer.
A positive test result may also have important implications for the family members:
- Individualshavingharmful BRCA1 or BRCA2 mutation may pass it to their offspring, even they have never developed cancer in their lifetime (Each child will have a 50% chance).
- Full sinblings of an Individualwhohas inherited a harmful BRCA1 or BRCA2 mutation has a 50% chance of having the mutation
- Negative result: A negative test results means that no pathogenic mutation was identified in BRCA1 and BRCA2 genes of the tested individual. However, testing negative for BRCA doesn’t necessarily mean the person will never develop cancer, the individual still fall in the general population risk of developing breast or ovarian cancer.
The meaning of a negative result partly depends also on an individual’s family history of cancer and whether BRCA1 and BRCA2 mutation is present in a blood relative
- If a 1st or 2nd degree relative of the tested individual carries a BRCA1 and BRCA2 mutation, a negative test result means that the person does not carry the mutation that is responsible for their family’s cancer risk (cannot pass the mutation to their offsprings). Such as test result is called true negative.
- Women predisposed to breast cancer based on family history but tested negative for BRCA1 and BRCA2mutations may consider more inclusive genetic testing called Advanced BRCA testing.
- Uncertain result: Sometimes, a genetic test finds a change in BRCA1 and BRCA2gene that has not been found to be associated with cancer. This type of results is described as uncertain or Variant of Uncertain Significance (VUS). These variants are mostly benign and most likely do not cause increased cancer risk. However, several reasons exist for calling a variant as VUS. These include;
- The effect of specific genetic alteration or gene function is not known
- There are insufficient genetic data to definitively confirm that the variant is associated with the risk of developing cancer
A Variant of Uncertain Significance (VUS) should not be used in clinical decision making (e.g. not clinically actionable).
Advanced BRCA TestingAdvanced BRCA testing are extended multi-gene panels recommended for follow-up after negative BRCA testing. It can either be added with BRCA1 and BRCA2 in the initial screening or opted after a BRCA 1/2 negative result. Though BRCA1 and BRCA2 are the major contributor for hereditary breast and ovarian cancer it has been reported that a large proportion of families segregating breast cancer alone are not caused by mutations in BRCA 1 or BRCA2.
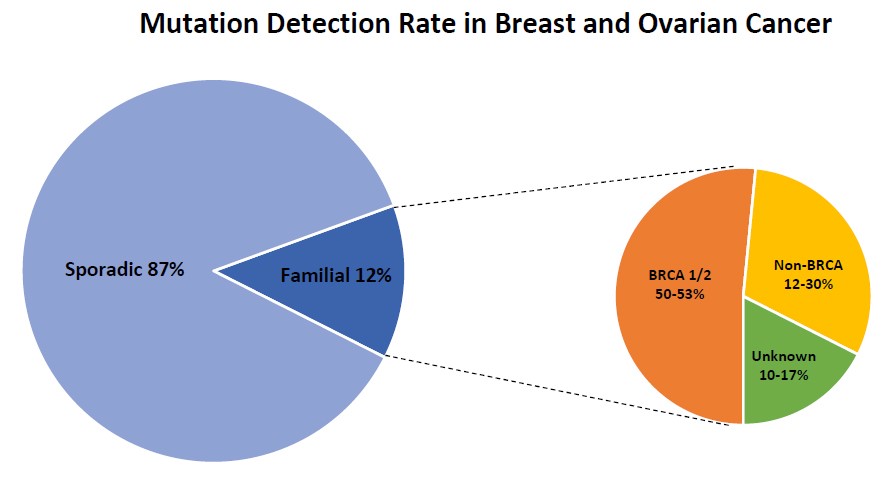
Individuals with a family history of breast cancer in closely related family members represent about 12.5% of all breast cancer cases. Within this group germline mutations in BRCA1 and BRCA2 confer an average 50% of the risk for breast cancer. However, several large-scale studies have identified additional 10-17 genes that may be otherwise be missed by testing BRCA1 and BRCA2 genes only. The diagnostic yield in these non-BRCA genes appear to be between 12-30%.

- NCCN Breast Cancer treatment Guidelines (http://screening.iarc.fr/doc/Breast_VIII.pdf)
- Guidelines for management of breast cancer (http://applications.emro.who.int/dsaf/dsa697.pdf)
- Clinical Practice Guidelines on Breast Cancer: ESMO (https://www.esmo.org/guidelines/breast-cancer)